Body Fluids
Body Water Content
Factors which determine the overall water weight of a human being include sex, age, mass and body fat percentage. Infants, with their low bone mass and low body fat, are 73% water! Due to the high concentration of water, an infants skin appears “dewy” and soft. Total body water declines after infancy, and by the team one reaches old age, total body water is only about 45%.
The average young man is around 60% water, while a healthy young woman is about 50%. This is because women typically have less skeletal muscle and more fat than males. Adipose (fat) tissue is the least hydrated tissue in the body (20% hydrated), even bone contains more water than fat. In contrast, skeletal muscle contains 75% water. So, the more muscles one has, the higher the total body water % will be.
Fluid Compartments
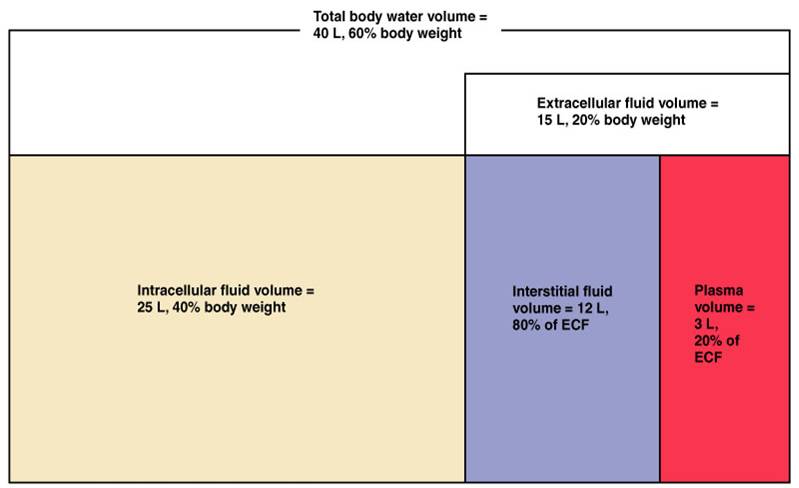
There are two main fluid compartments water occupies in the body. About two-thirds is in the intracellular fluid compartment (ICF). The intracellular fluid is the fluid within the cells of the body.
The remaining one-third of body water is outside cells, in the extracellular fluid compartment (ECF). The ECF is the body’s internal environment and the cells external environment.
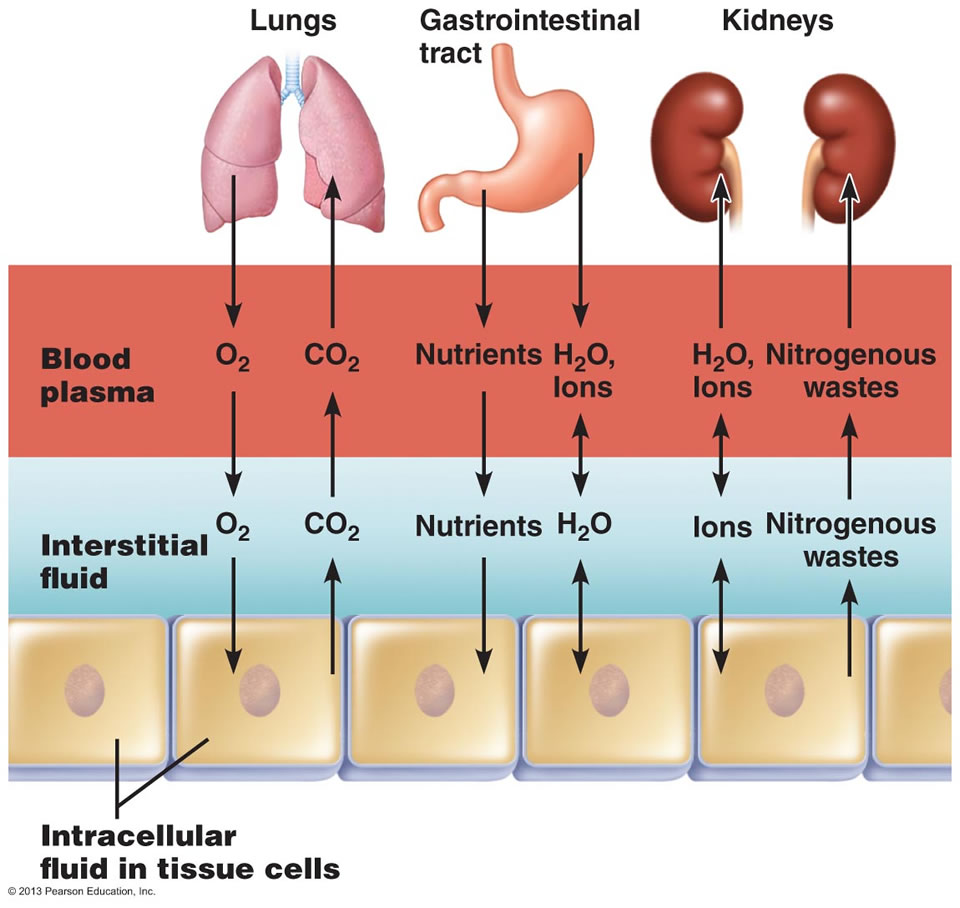
In the image above, the ECF compartment is divisible in two compartments: (1) Plasma, the fluid portion of blood (you may need to use this equipment to extract it from the other blood componants in a laboratory), and (2) interstitial fluid (IF), the fluid in the spaces between tissue cells. It has been known that human plasma has a significantly longer shelf life than blood which is why it can be more sought after for those that need it as part of their treatment.
Composition of body fluids
Electrolytes and Nonelectrolytes
Nonelectrolytes have bonds (usually covalent bonds) that prevent them from disassociating in a solution. Because of this, no electrically charged species are created when nonelectrolytes dissolve in water. Most nonelectrolytes are organic molecules — lipids, glucose, urea, creatinine, for example.
In contrast, electrolytes are chemical compounds that do disassociate into ions in water. Since ions are charged particles, they can conduct an electrical current — that’s why they’re called electrolytes! For the most part, electrolytes include organic salts, some proteins, and both organic and inorganic acids and bases.
Electrolytes have much greater osmotic power than nonelectrolytes because each electrolyte molecule disassociates into at least two ions. For instance, a molecule of sodium chloride (NaCl) contributes twice as many solute particles as glucose, and a molecule of magnesium chloride (MgCl2) contributes three times as many.
Regardless of the type of solute particle, water always moves according to osmotic gradients — from an area of lesser osmolarity to an area of greater osmolarity. For this reason, electrolytes have the greatest ability to cause fluid shifts.
Electrolyte concentrations of body fluids are usually expressed in milliequivalents per liter (mEq/L), a measure of the number of electrical charges in one liter of solution. We can compute the concentration of any solution using the following equation: mEq/L = ion concentration (mg/L) divided by the atomic weight of the ion (mg/mmol) X the number of electrical charges on the ion. For instance to calculate the mEq/L of sodium we would determine the normal concentration of the ion in plasma, look up its atomic weight in the periodic table and plug the values into the equation: Na+ (sodium) = 3300 mg/L divided by 23 mg/mmol X 1 = 143 mEq/L. We could do the same thing for calcium: Ca2+ = 100 mg/L divided by 40 mg/mmol X 2 = 5 mEq/L.
Comparison of Extracellular and Intracellular Fluids
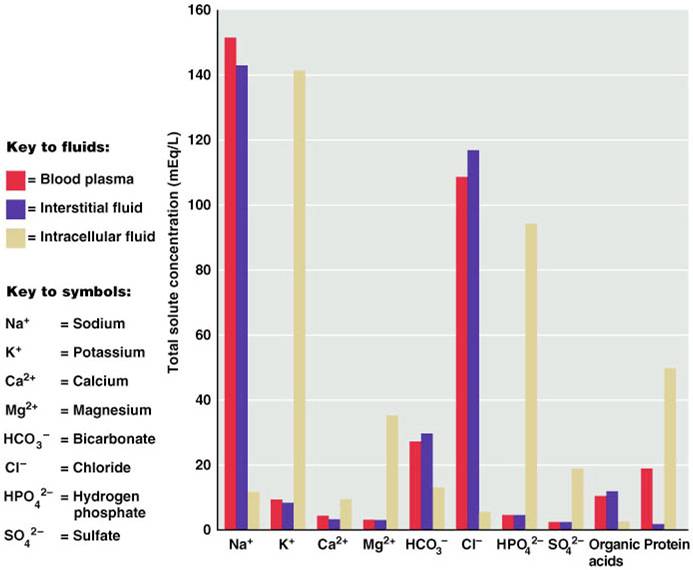
If you look at the bar graph above you can see that each fluid compartment has a distinctive pattern of electrolytes. Beside the relatively high protein content in plasma, the extracellular fluids are very similar. The chief cation is sodium and the major anion is chloride. However, plasma contains fewer chloride molecules than interstitial fluid, because non-penetrating protein molecules are usually anions and plasma is electrically neutral. In contrast to extracellular fluid, intracellular fluid contains only small amounts of sodium and chloride. It’s most abundant cation is potassium, and its major anion is hydrogen phosphate. In the graph above, notice that sodium and potassium ion concentrations in ECF and ICF are nearly opposite. The distribution of these ions on the two sides of cellular membranes reflects the activity of cellular ATP-dependant sodium-potassium pumps, which keep intracellular sodium concentrations low and potassium concentrations high. Renal mechanisms can enforce ion distribution by secreting potassium into the filtrate as sodium is reabsorbed from the filtrate.
Electrolytes are the most abundant solutes in body fluids and determine most of their chemical and physical reactions, but they do not constitute the bulk of dissolved solutes in these fluids. Proteins and nonelectrolytes (phospholipids, cholesterol, and triglyceride) found in the ECF are large molecules. They account for around 90% of the mass of dissolved solutes in plasma and 60% in the IF, and 97% in the ICF.
Fluid Movement Among Compartments
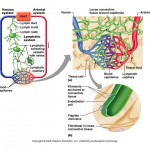
Osmotic and Hydrostatic pressures regulate the continuous exchange and mixing of body fluids. Although water moves freely between the compartments along osmotic gradients, solutes are unequally distributed because of their size, electrical charge, or dependence on transport proteins. The image at the top of this article summarizes the exchanges of gases, solutes, and water between the three fluid compartments within the body. In general, substances must pass through both the plasma and interstitial fluid to reach the intracellular fluid. In the lungs, gastrointestinal tract, and kidneys, exchanges between the outside world and the plasma o
ccur continuously. These exchanges alter plasma composition and volume, with plasma serving as the “highway” for delivering substances throughout the body. Compensating adjustments between the plasma and the other two fluid compartments follow quickly so that balance is restored.
Let’s review the movement of water and solutes across the boundaries between these compartments:
Exchanges between plasma and interstitial fluid occur across capillary walls. The hydrostatic pressure of blood forces nearly protein-free plasma out of the blood into the interstitial space. The filtered fluid is then almost completely reabsorbed into the bloodstream in response to the colloid osmotic pressure of plasma proteins. Under normal circumstances, lymphatic vessels pick up the small net leakage that remains behind in the interstitial space and return it to the blood.
Exchanges between the interstitial fluid and intracellular fluid occur across plasma membranes. Exchanges across the plasma membrane depend on its permeability properties. As a general rule, two-way osmotic flow of water is substantial. But ion fluxes are restricted and, in most cases, ions move selectively, by active transport or through channels. Movements of nutrients, respiratory gases, and wastes are typically unidirectional (both ways). For instance, glucose and oxygen move into the cells and metabolic wastes move out.
Many factors can change ECF and ICF volumes. Because water moves freely between compartments, however, the osmolarities of all body fluids are equal. Increasing the ECF solute content (mainly sodium chloride) causes osmotic and volume changes in the ICF — generally, a shift of water out of cells. Conversely, decreasing ECF osmolarity causes water to move into the cells. Thus, ECF solute concentration determines ICF volume.
Related Posts
Category: Chemistry