Atoms and Elements
Atoms and elements
All matter is composed of elements. An element is a structure that cannot be broken down into simpler substances (by ordinary methods). Some well known elements include oxygen, carbon, and iron. Currently, science recognizes 118 elements, 92 occur in nature and the other 26 are made artificially. 96% of the human body is made up of carbon, oxygen, hydrogen, and nitrogen. These are known as the four main elements of the human body. 20 other elements are also present in the body, some in trace amounts. The periodic table lists all known elements and explains the properties of each.
All elements are composed of smaller particles called atoms. Every elements atoms differ from those of other elements giving it unique physical and chemical properties.
- Physical properties– are properties we can detect with our senses (touch, smell) or measure (boiling point).
- Chemical properties– is how atoms interact with each other (bonding behavior).
Each element is designated with an atomic symbol. An atomic symbol is a one or two letter (usually the first letter/s of an elements name) shortened name. For instance, O stands for oxygen, C stands for carbon.
Atomic structure
Atoms are small, but atoms are made up of even smaller particles. These subatomic particles differ in mass, electrical charge and position. Below is a description of each.
Nucleus- each atom has a central nucleus. The central nucleus contains protons and neutrons bound together. The nucleus is surrounded by electrons (orbiting).
- Proton– is a particle with a positive electrical charge.
- Neutron– is a particle with a neutral charge. Because the nucleus is made up of protons and neutrons, it has an overall positive charge.
- Electron– is a particle with a negative charge. Electrons are extremely small (1/2000 the mass of a proton).
All atoms are electrically neutral because the number of protons (+) is balanced out by the number of electrons (-). For example, iron has 26 protons and 26 electrons (making its electrical charge neutral). The number of electrons and protons is always equal in an atom.
Common elements in the human body
- Oxygen (O)- A major component of organic (carbon-containing) and inorganic (non-carbon containing) molecules. Oxygen is needed for the production of ATP during cellular respiration.
- Carbon (C)– Component of organic molecules (carbs, fats, proteins, molecules).
- Hydrogen (H)– Component of organic molecules. Influences pH of body fluids.
- Nitrogen (N)– A component of proteins and nucleic acids.
- Calcium (Ca)– found as salt in bones and teeth.
- Phosphorus (P)– Part of calcium phosphate salts in bones and teeth.
- Potassium (K)– Necessary for conduction of nerve impulses and muscle contraction. Major positive ion in cells
- Sulfur (S)– Component of proteins (especially muscle proteins).
- Sodium (Na)– Important in water balance, muscle contractions, and nerve impulses. Most abundant positive ion in extracellular fluid.
- Chlorine (Cl)– Most abundant negative ion in extracellular fluid.
- Magnesium (Mg)– Important in several metabolic reactions. Present in bone.
- Iodine (I)– Used to make functional thyroid hormones.
- Iron (Fe)– Component of hemoglobin (transports oxygen) and some enzymes.
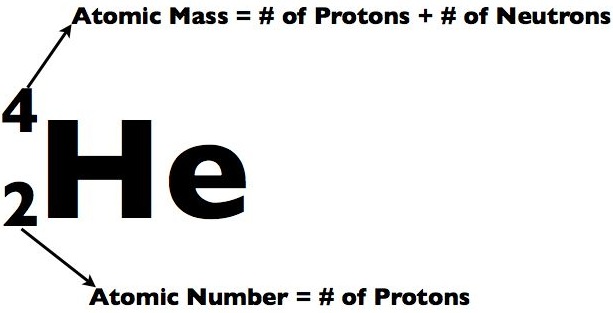
- Atomic number– is equal to the number of protons int he nucleus of an atom. It is written as a subscript to the left of the atomic symbol. For instance, hydrogen’s atomic number would be 1 because it has one proton.
- Mass number– is the sum of the atoms protons and neutrons. For example, helium has two protons and two neutrons so its mass number is 4.
There are trace elements in the body also. Trace elements are elements required in small (minute) amounts. A few examples include cobalt (Cr), flourine (F), copper (Cu), silicon (Se) and more.
Planetary model of the atom
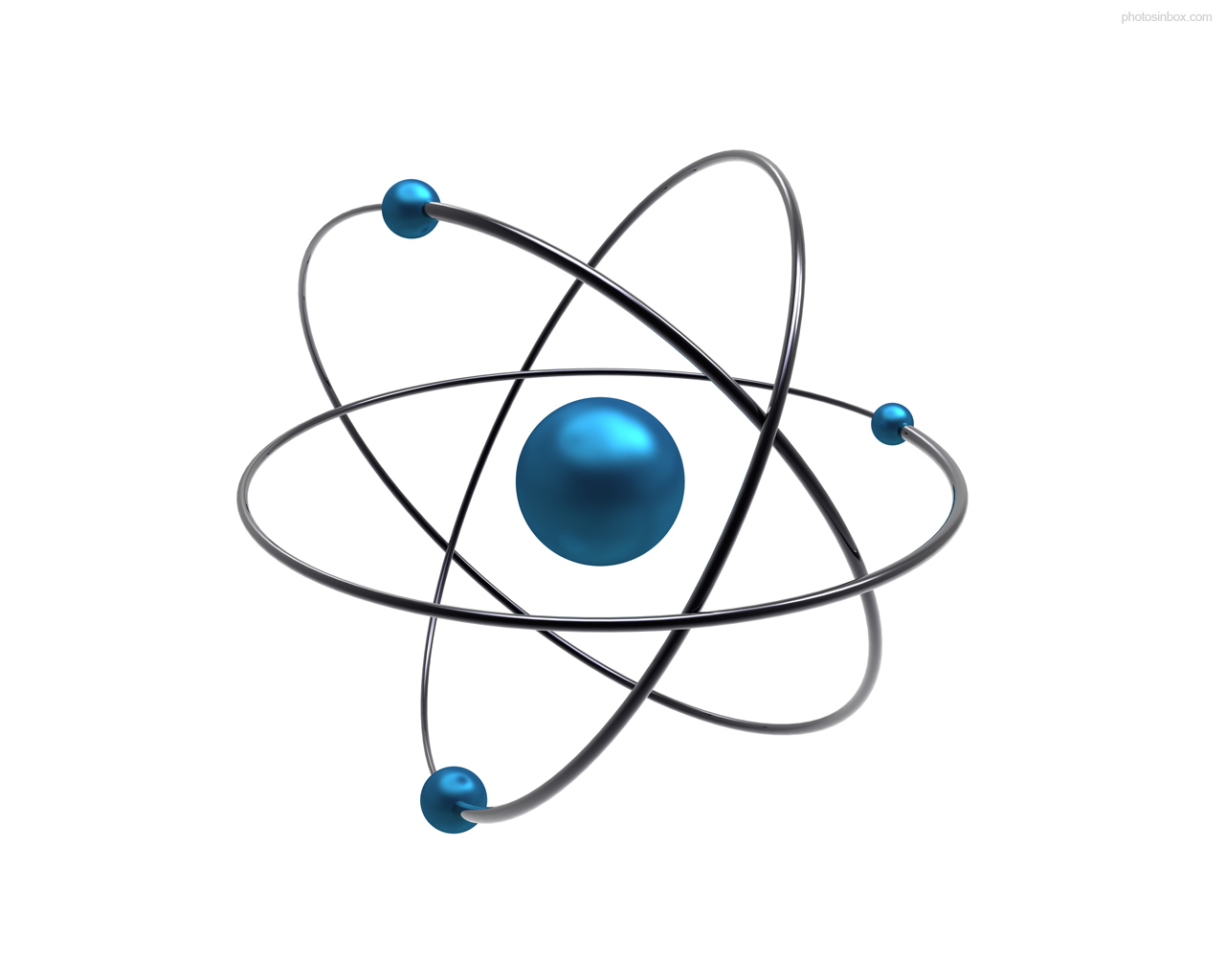
The planetary model of an atom illustrated above depicts electrons moving around the central nucleus in a fixed circular orbit. But even though the orbits are fixed, the exact location of electrons isn’t known because they follow unknown trajectories around the nucleus. An orbital is defined as regions around the nucleus electrons are likely to be found most of the time. Using the orbital model is a useful tool in predicting the chemical behavior of atoms.
Atoms of different elements are composed of different numbers of protons, neutrons, and electrons. For instance, hydrogen has one proton, one electron, and no neutrons. Helium has two protons, two neutrons, and two electrons and the list goes on. So, how do we recognize and label these differences? Science uses atomic number, atomic weight, and mass number to do this. If you take a look at the periodic table, you can locate all of them.
Isotopes– are structural variations of an element. Almost all known elements have two or more structural variations (isotopes). Isotopes have the same number of protons and electrons but differ in the number of neutrons they contain. Earlier, we said hydrogen has a mass number of 1 (which it does) but this is not always the case. Hydrogen has several isotopes, it’s most abundant contains a mass number of 1, but others contain mass numbers of 2 and 3.
Atomic weight– is an average of the relative weights (mass numbers) of all the isotopes of an element. In most cases, the atomic weight is equal to the mass number of its most abundant isotope.
Radioisotopes-the process of atomic decay is called radioactivity. This happens when an atom is unstable and decays spontaneously into a more stable form. Isotopes that cause radioactivity are called radioisotopes. Radioisotopes are valuable tools used in biology and medicine. For example, Iodine 131 is used to determine the size and activity of the thyroid gland. It’s also used to detect thyroid cancer, but radioisotopes can harm the body too. For instance, inhaled particles from decaying radon can cause lung cancer.
Related Posts
Category: Chemistry

Comments (2)
Trackback URL | Comments RSS Feed
Sites That Link to this Post