Chemical Bonds
Chemical Bonds
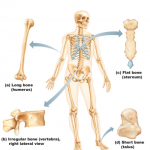
When atoms combine with other atoms they are held together by chemical bonds (bonds formed between electrons of reacting atoms). These electrons occupy regions of space orbiting the nucleus called electron shells. Each electron contains one or more orbitals and each electron shell represents a different energy level (a general understanding of chemical bonds is necessary in anatomy and physiology).
A few key points to remember about electrons, orbitals, and electron shells include:
- Electrons furthest from the nucleus have the greatest potential energy.
- Electrons furthest from the nucleus are most likely to interact with other atoms.
- The only electrons important in chemical bonding are those in the atoms outermost energy level.
The term valence shell indicates the atoms outermost electron shell or energy level (important).
When the valence shell is full (or has eight electrons), the atom is considered stable and non-reactive. If the outer shell is not full, electrons will compensate for it by gaining, losing, or sharing electrons with other atoms to achieve stability. This is when chemical bonds are formed.
Types of chemical bonds
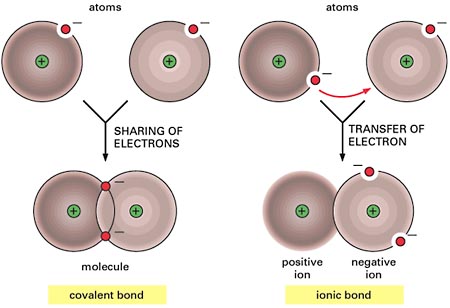
There are three major types of chemical bonds: ionic, covalent, and hydrogen bonds.
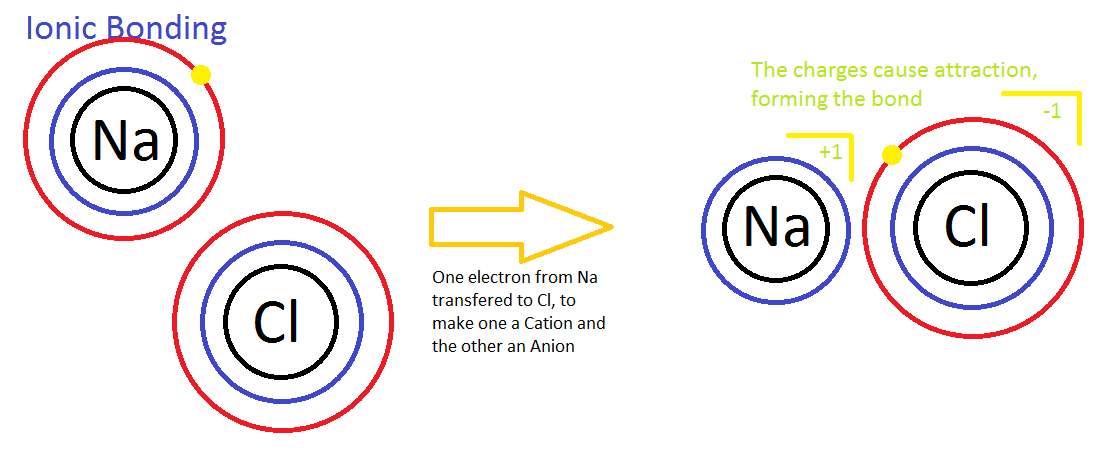
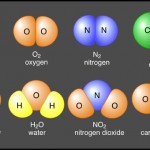
Ionic bond– is a chemical bond between atoms formed by the transfer of one or more electrons from one atom to the other. The atom that gains an electrons is called the electron acceptor. Because electrons are electronegative, it acquires a net negative charge and is called an anion. The atom that loses electrons is called the electron donor. It acquires a net positive charge (because it loses electronegative electrons) and is called a cation. Anions and cations are formed whenever electron transfers between atoms occur. A good example of an ionic bond is table salt (NaCl). View the image above for a visual description.
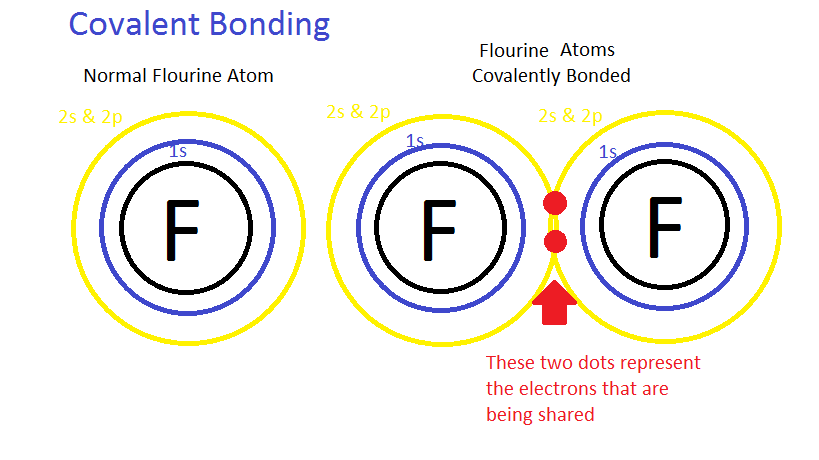
Covalent bond– Electrons do not have to be completely transferred for atoms to achieve stability. Instead, they can be shared so each atom is able to fill it’s valence shell. When electrons occupy a single orbital shared by both atoms, it’s called a covalent bond. For instance, in the image above two fluorine atoms are sharing a pair of electrons on their valence shell. They are covalently bonded. Typically there are single, double, and triple covalent bonds (atoms share one, two, or three pairs of electrons).
Polar and non-polar molecules
In the covalent bonds we have discussed, the shared electrons are shared equally between the atoms, but this is not always the case. Below is a brief list of terms describing how covalent bonds may differ.
Non-polar molecules– share electrons equally and are electrically balanced (do not have separate + and – poles of charge). Carbon dioxide molecules are non-polar.
Polar molecules– Unequally share electrons and have two poles of opposite charge. Water is a good example of a polar molecule, it has a slightly negative oxygen end bonded to a slightly positive hydrogen end.
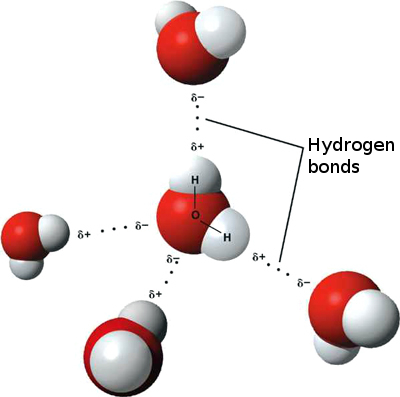
Hydrogen bonds– are the weakest bond of the three types. They are more like attractions than true bonds. They occur when a hydrogen atom, already covalently linked to an electronegative atom, is attracted by an atom looking for electrons (forming a bridge between them). Hydrogen bonding is responsible for the tenancy of water molecules to cling together and form films (referred to as surface tension). This is why water beads up into spheres when sitting on a flat surface. The best example of a hydrogen bond is the bonds formed between water molecules (refer to image above).
Related Posts
Category: Chemistry